Student teachers at Ball State University are required to complete an in-depth unit long data driven analysis of their student teaching called Learning Assessment Model Project (LAMP). I chose to run the project during the stoichiometry unit. Stoichiometry is a unifying topic of chemistry. It synthesizes previously taught topics of writing chemical formulas, calculating molar masses, performing molar conversions, and balancing chemical reaction equations so that students may apply prior knowledge to its fullest use. Stoichiometry is rigorous and challenging for students. My aim was to make this topic both accessible and useful for my students by relating it to their everyday lives. We chose the stoichiometry unit to run our LAMP project in as most students, except possibly those who have taken integrated chemistry and physics, have no experience with stoichiometry. We wanted to see the direct impact that our teaching had upon students. The following is a summary of the project, its outcome and conclusions drawn from it.

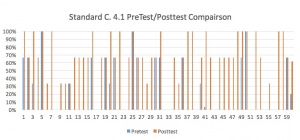
IN Standard C.4.4 was the essential standard assessed by the unit: Using a balanced chemical equation, calculate the quantities of reactants needed and products made in a chemical reaction that goes to completion. A formative pretest, three laboratories, one teamwork conversion assignment, a quizlet, a project and a summative end of unit exam were performed during the sixteen day unit.
My first ever attempt at differentiating instruction occurred after the midweek quizlet, when I divided students into ability groups based upon their performance on the formative assessment. If students scored a 3-4-5 out of 5, they were given the list of five types of chemical reactions and were asked to research and find examples of them, in hopes that this would be a gateway of thought into the stoichiometry project. Students who scored a 1 or 2 completed more practice stoichiometry problems with me so that we could focus on errors in their calculations while building confidence in their problem solving skills.
Once I tried differentiation, I was hooked on it and made sure to include a differentiated activity at least once a week for the duration of the semester, and students came to expect them. I didn’t use other informal assessments, such as bell ringers or exit slips, until the next unit, and that is one of my regrets for this unit project. When I completed this project in February I still felt that such activities were a waste of instructional time, but once I realized how valuable they are as a quick pulse check on student understanding, I integrated their use more frequently. My second regret is that I focused too much on the mathematics of the unit, as at the time I believed that if students could do the math, they would understand the chemistry. I certainly missed the mark on that, and it wasn’t until I attended HASTI and I sat in on a session using the Three Levels of Chemistry that includes a macro and micro definitions, that it even occurred to me how I should ensure clear conceptual understanding of concepts that I cover. I went on to do this much more deliberately in subsequent units.
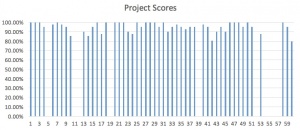
The students who performed the initial stoichiometry research assignment got a brief glimpse into types of chemical reactions and their use in life. The stoichiometry project was a more in-depth study of a single type of reaction in which students found a particular interest. One student had a special interest in rockets, so he chose combustion of rocket fuel as his topic. Some of the students who are considering the medical field as a future profession chose pharmaceutical synthesis reactions, and realized how just how complex those mechanisms are. Other students chose combustion reactions involving sport or automobile objects, while yet others chose to look at components found in personal care products. The goal of the project was for students to find something personally interesting to them and to see how it was generated by or contributed to a chemical process. I was impressed at their creativity, and proudly displayed as many projects as I could on our classroom bulletin board.
The focus of the project was equally balanced between accurate calculations based upon their balanced chemical reaction equation, and the neatness of the project and included pictures, illustrations or diagrams. I liked how the LAMP project required me to insert a unit project, as I typically would not have added one. Students need more kinds of assessment, and need to be allowed to tap into their interests and creativity, and this assignment allowed for all of that. Here is the rubric used to grade the unit projects:
We used our labs to prove how important having sound techniques is. We relied upon group work, both for labs and problem solving at the board and on worksheets. We used think-pair-share activities to kick off discussions and to activate previous knowledge. Assignments included:
Limiting reagent inquiry lab #1
Stoich lab cpd determination #2
Teamwork Stoichiometry Practice
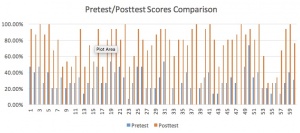
Taking data, interpreting it, using it to guide instruction and make conclusions was the main purpose of this project. As such, a huge amount of data was compiled and analyzed. Three sections of Chemistry I were taught the unit concurrently, for a total of 59 students. Scores were converted to percentages, and the final column in each graph is for the class average. Here is a sampling of some of the comparison graphs:
Multiple InTASC standards were covered by this unit plan, as they should have been. InTASC 1 was used in the pretest/posttest portion data collection portion of the unit. Considering differentiating my teaching to honor learning styles is a component of InTASC 2. Cooperative learning and engagement in activities aligns with InTASC 3 & 4. Introducing brand new content falls under InTASC 5’s umbrella. The creativity and critical thinking required for the unit project and its rubric fulfill InTASC 6’s assessment requirements. The relatability of the project to real life is an aspect of InTASC7. Finally, modeling the stoichiometry organizer’s use completes InTASC 8. HLPs used included 1-2-3-4-6-9-10-11-13-14-15-16-19.
The fact that so many InTASC standards and HLPs were used during the duration of this unit tells me that my teaching starting off in the right direction. I attempt to use data to drive my classroom decision making, and I enjoyed how this project forced me to do so on a larger scale. It was a huge undertaking, but a worthwhile one to see the true impact of my teaching on student learning.